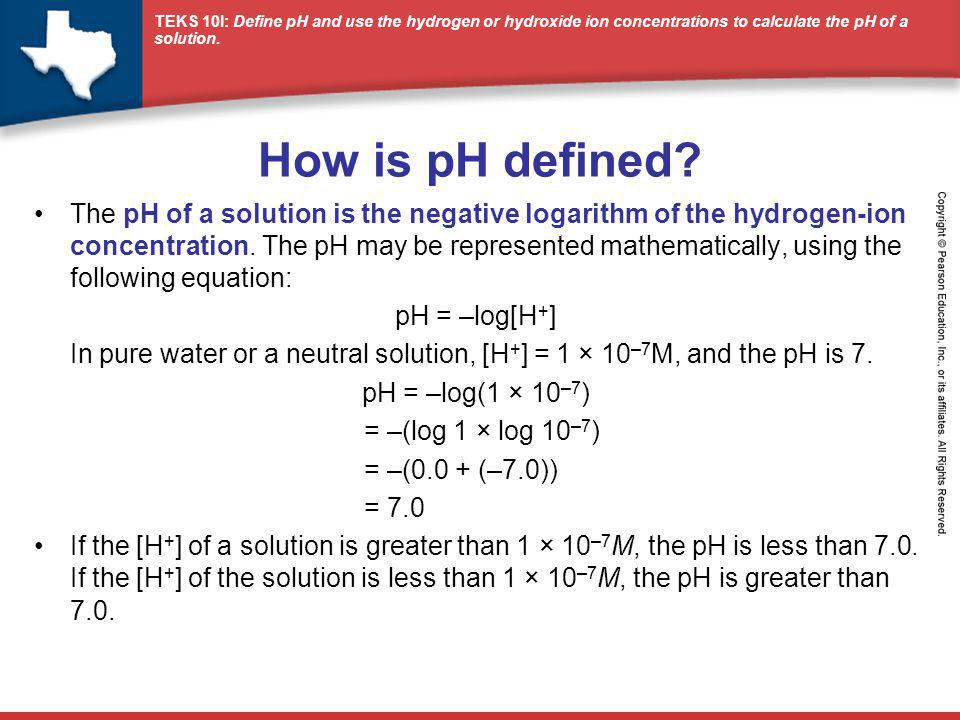
How is pH defined? The pH of a solution is the negative logarithm of the hydrogen-ion concentration. The pH may be represented mathematically, using the. - ppt video online download
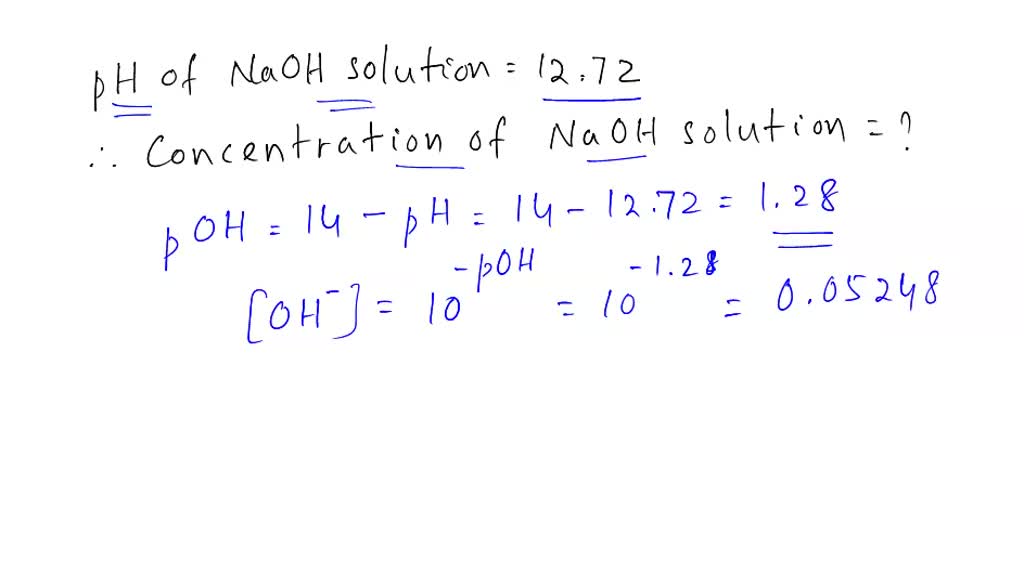
SOLVED: Calculate the concentration of an aqueous solution of NaOH that has a pH of 12.72. Express your answer using two significant figures.

pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems - YouTube
![The pH of a solution is 8.6. Calculate the OH^(-) ion concentration pH=8.6 pOH=5.4 -log[OH^(-)]=10^(-5.4) [OH^(-)]=10^(-6)xx10^(0.6)=10^(-6)xx anto log 0.6 [OH^(-)]=3.98xx10^(-6) The pH of a solution is 8.6. Calculate the OH^(-) ion concentration pH=8.6 pOH=5.4 -log[OH^(-)]=10^(-5.4) [OH^(-)]=10^(-6)xx10^(0.6)=10^(-6)xx anto log 0.6 [OH^(-)]=3.98xx10^(-6)](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/644422609_web.png)
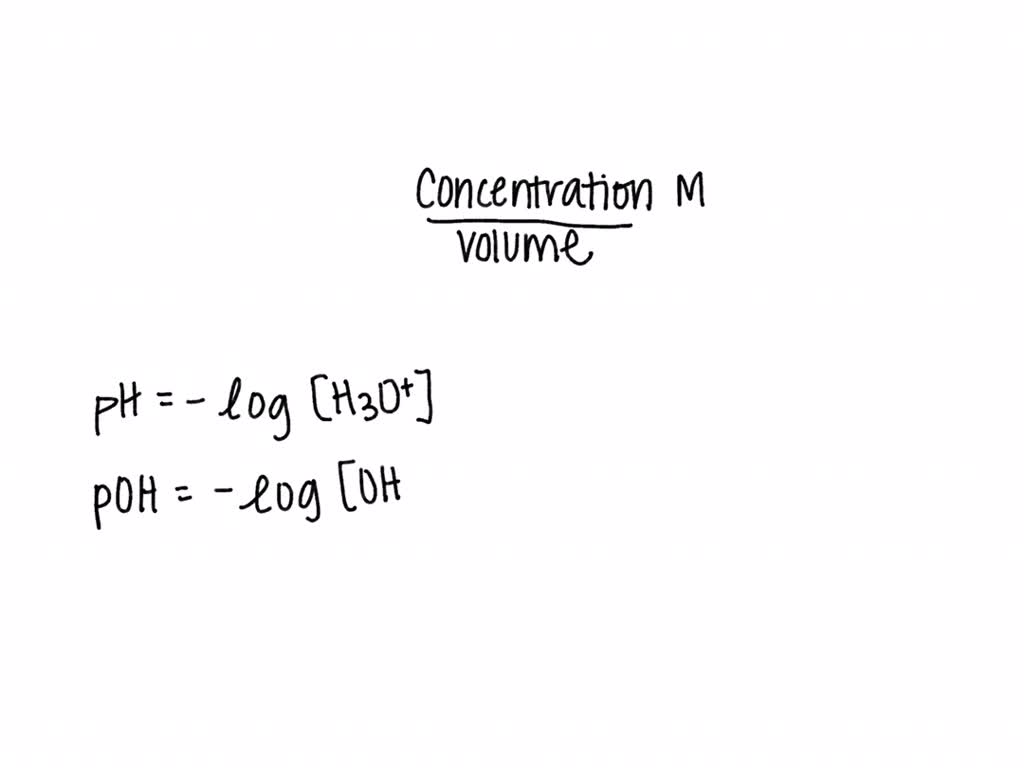
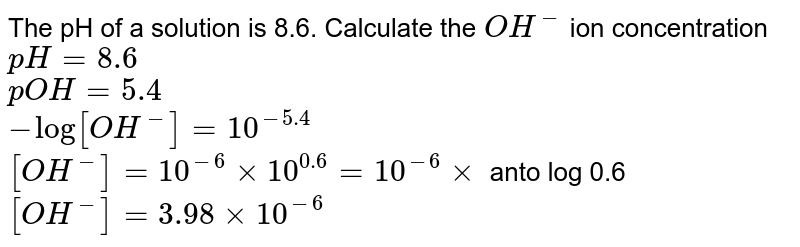



![Given [H+] or [OH-], Calculate pH & pOH - YouTube Given [H+] or [OH-], Calculate pH & pOH - YouTube](https://i.ytimg.com/vi/ghIYaqo0Ycc/maxresdefault.jpg)


![Solved Q. Calculate the hydronium ion concentration, [H3O+], | Chegg.com Solved Q. Calculate the hydronium ion concentration, [H3O+], | Chegg.com](https://d2vlcm61l7u1fs.cloudfront.net/media%2Fc0b%2Fc0bdec03-36e7-4e75-9728-5bb80e1953c4%2FphpMPXgS8.png)



![Solved Calculate the hydroxide ion concentration, [OH-], for | Chegg.com Solved Calculate the hydroxide ion concentration, [OH-], for | Chegg.com](https://media.cheggcdn.com/media/3ff/3ff607b6-3db1-436d-a7e8-12fbd50c976f/php00qsUS)
