The redox language in neurodegenerative diseases: oxidative post-translational modifications by hydrogen peroxide | Cell Death & Disease
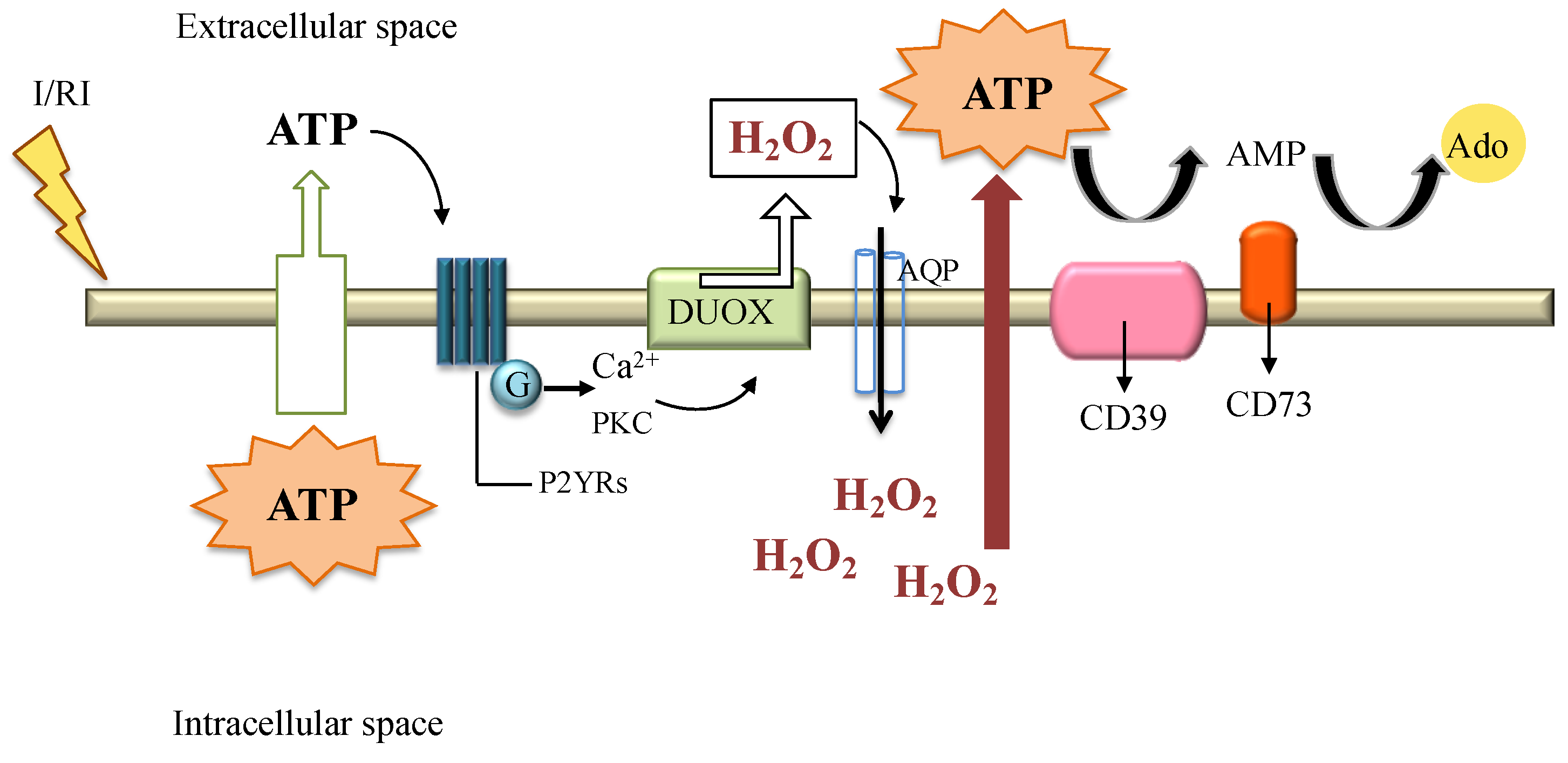
Cells | Free Full-Text | The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells

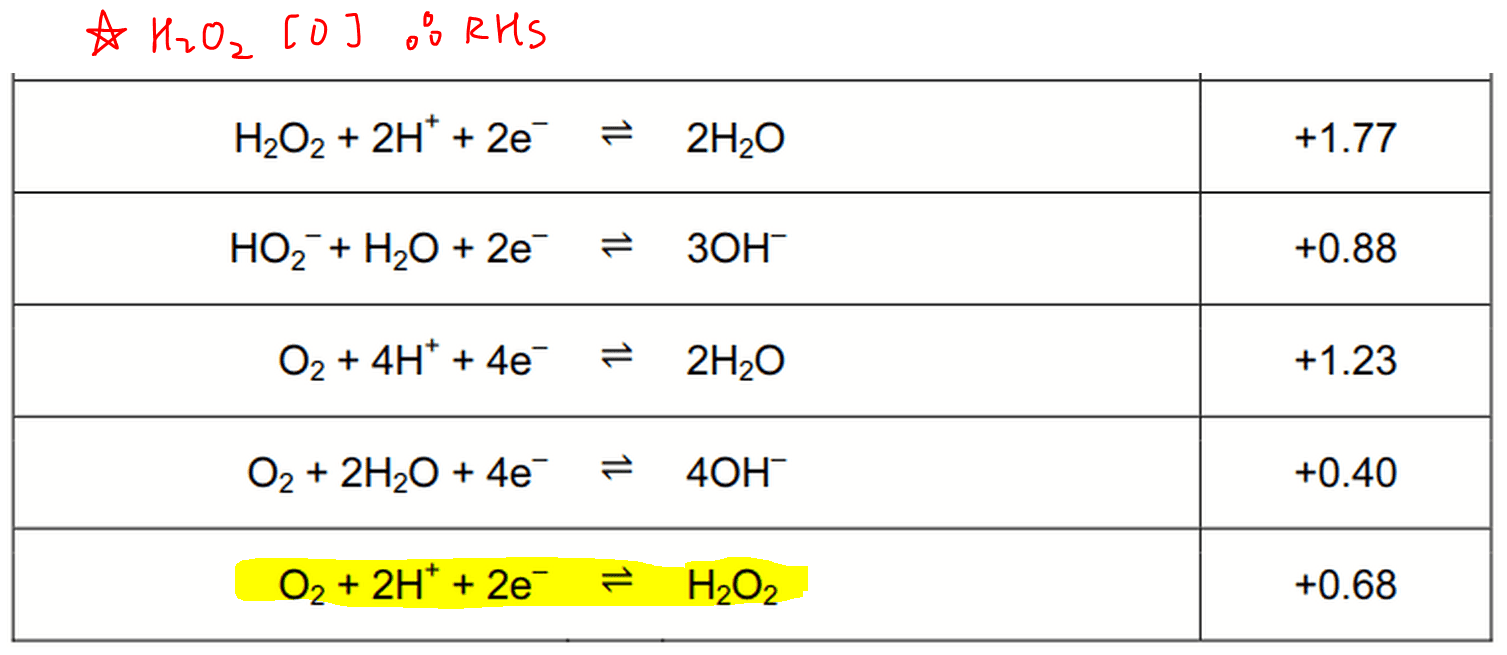
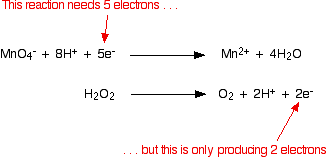
electrochemistry - How do I determine which is the reduction reaction in a hydrogen peroxide and potassium permanganate solution? - Chemistry Stack Exchange

While sulphur dioxide and hydrogen peroxide can act as oxidising as well as reducing agents in..... - YouTube
![PDF] Decomposition of hydrogen peroxide - kinetics and review of chosen catalysts | Semantic Scholar PDF] Decomposition of hydrogen peroxide - kinetics and review of chosen catalysts | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f2948b1ccb4f9f878523a741f22c7a299fa747b2/2-Figure1-1.png)
PDF] Decomposition of hydrogen peroxide - kinetics and review of chosen catalysts | Semantic Scholar
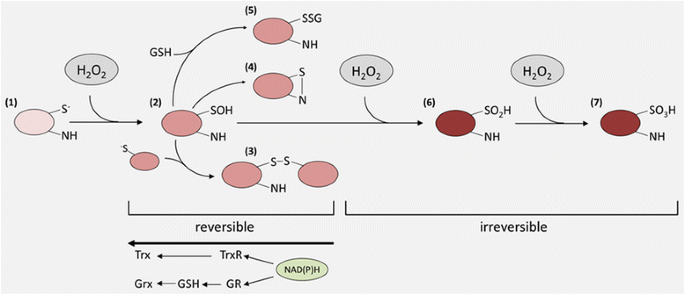
Hydrogen peroxide – production, fate and role in redox signaling of tumor cells | Cell Communication and Signaling | Full Text

Localized Redox Relays as a Privileged Mode of Cytoplasmic Hydrogen Peroxide Signaling | PedroAbranches

The redox reaction involving the reducing power of hydrogen sulphide is: S + 2H^ + + 2e^ - → H2S, E^ ∘S/H2S = + 0.14V Two other half equations are: Fe^3 + +
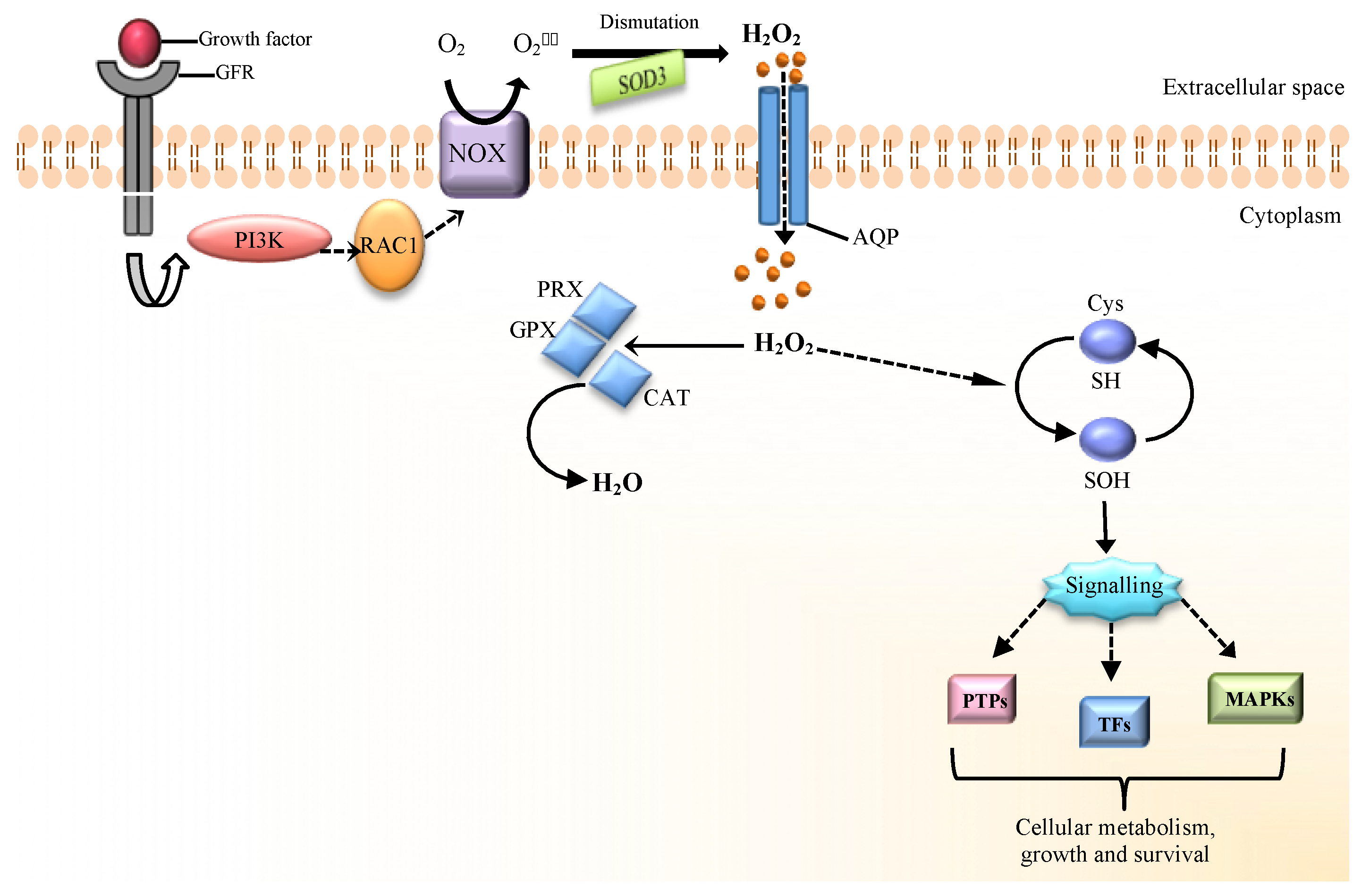
Cells | Free Full-Text | The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells
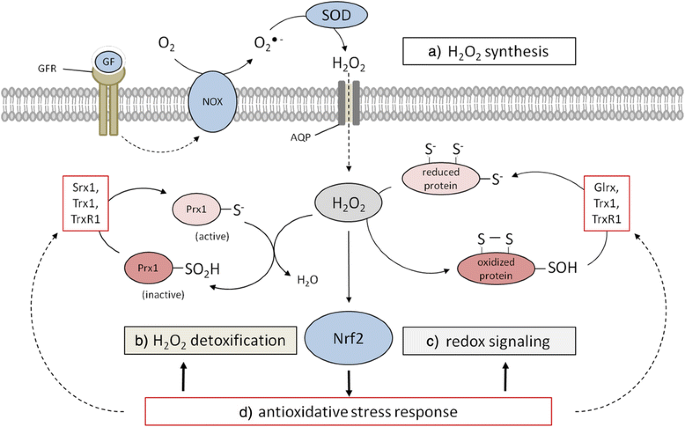
Hydrogen peroxide – production, fate and role in redox signaling of tumor cells | Cell Communication and Signaling | Full Text

Redox initiation reaction with the ascorbic acid/hydrogen peroxide system. | Download Scientific Diagram
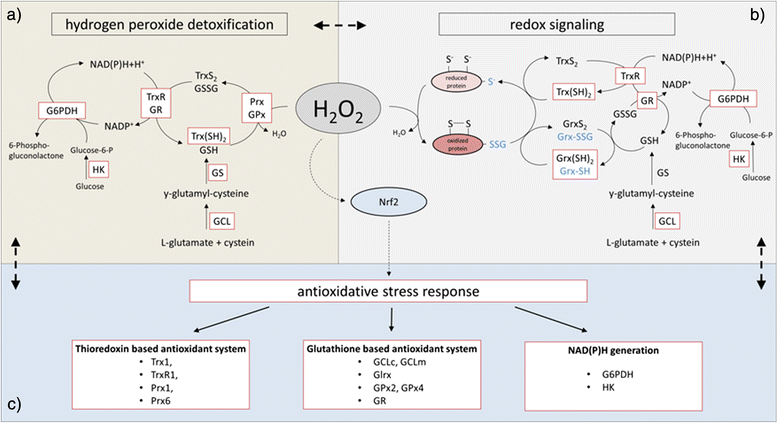
Hydrogen peroxide – production, fate and role in redox signaling of tumor cells | Cell Communication and Signaling | Full Text

SOLVED: The concentration of hydrogen peroxide solution can be found by a redox titration with acidified potassium permanganate solution: It was found that 1SmL of the potassium permanganate solution reacted with 32mL

Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress - ScienceDirect
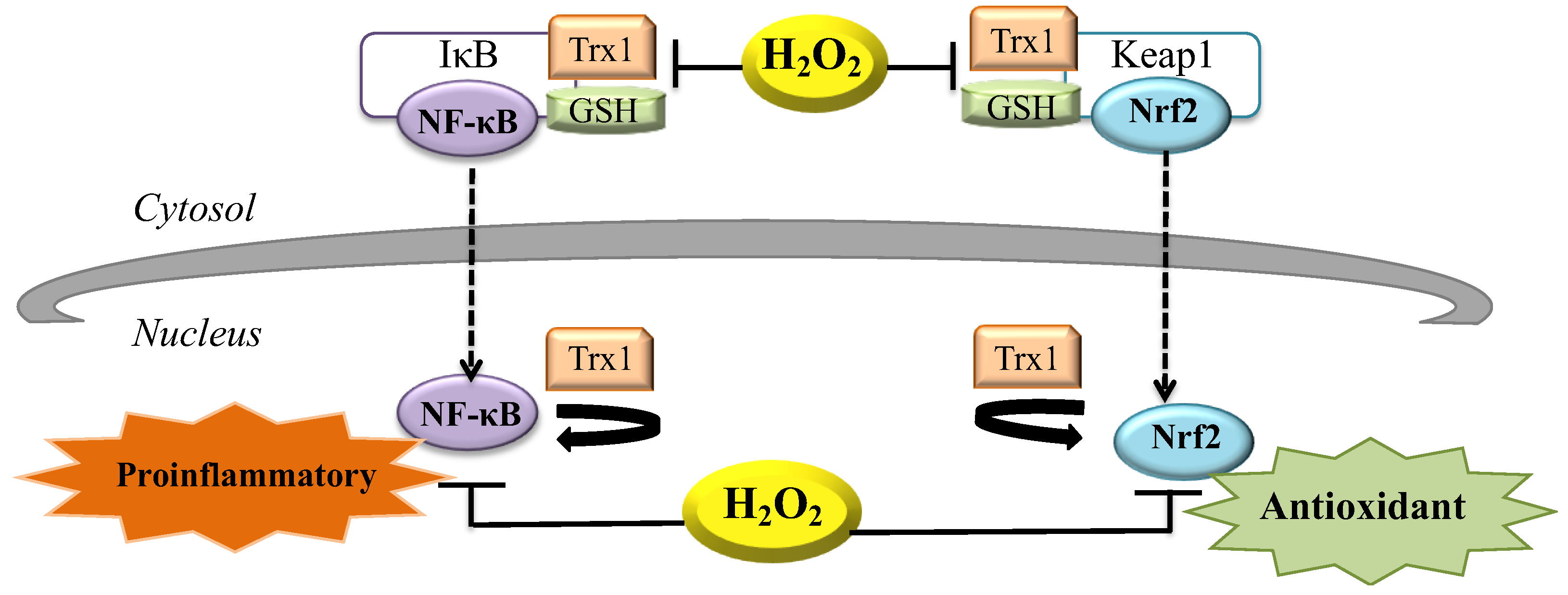
Cells | Free Full-Text | The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells

The oxidation-reduction reactions of hydrogen peroxide at inert metal electrodes and mercury cathodes - Transactions of the Faraday Society (RSC Publishing)