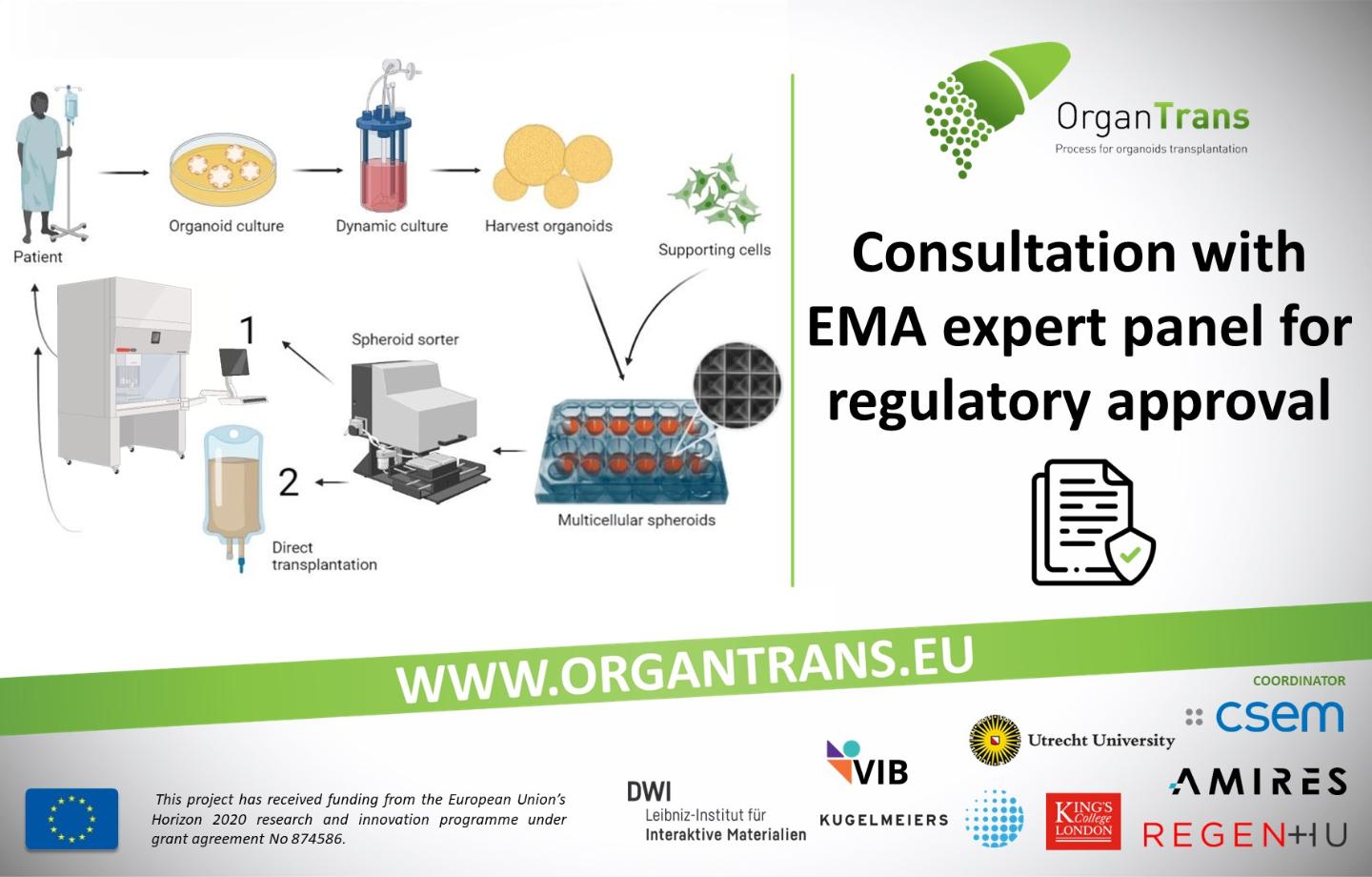
Recommendations for the implementation of the exemptions to the labelling and package leaflet obligations in the centralised pro

Europe - Joint Strategy sets direction for EMA and EU Medicines Regulatory Agencies to 2025 - RIS.WORLD

Novidades 20 de Outubro de 2020 - APREFAR - Associação dos Profissionais de Registos e Regulamentação Farmacêutica